
HL Paper 2
Two hydrides of nitrogen are ammonia and hydrazine, . One derivative of ammonia is methanamine whose molecular structure is shown below.

Hydrazine is used to remove oxygen from water used to generate steam or hot water.
The concentration of dissolved oxygen in a sample of water is .
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Determine the enthalpy change of reaction, , in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
The standard enthalpy of formation of is . Calculate the enthalpy of vaporization, , of hydrazine in . (If you did not get an answer to (f), use but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from of the sample.
Calculate the volume, in , of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = at SATP.)
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
Predict whether the molecules PF3 and PF5 are polar or non-polar.
State the type of hybridization shown by the phosphorus atom in PF3.
Bonds can be formed in many ways.
The equilibrium for a mixture of NO2 and N2O4 gases is represented as:
2NO2(g) N2O4(g)
At 100°C, the equilibrium constant, Kc, is 0.21.
Bonds can be formed in many ways.
Discuss the bonding in the resonance structures of ozone.
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
State the hybridization of the carbon I and II atoms in but-2-ene.
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows.
Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
Outline how two enantiomers can be distinguished using a polarimeter.
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR
State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
Some physical properties of molecular substances result from the different types of forces between their molecules.
Resonance structures exist when a molecule can be represented by more than one Lewis structure.
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.

Deduce, giving a reason, the more likely structure.
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.
Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
Deduce the average oxidation state of carbon in butanoic acid.
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State a suitable reagent for the reduction of butanoic acid.
Deduce the product of the complete reduction reaction in (e)(i).
The overall equation for the production of hydrogen cyanide, HCN, is shown below.
CH4 (g) + NH3 (g) +O2 (g) → HCN (g) + 3H2O (g)
State why NH3 is a Lewis base.
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer solution.
Sketch the shape of one sigma () and one pi () bond.
Identify the number of sigma and pi bonds in HCN.
State the hybridization of the carbon atom in HCN.
Suggest why hydrogen chloride, HCl, has a lower boiling point than hydrogen cyanide, HCN.
Explain why transition metal cyanide complexes are coloured.
There is concern about damage done to the ozone layer in the stratosphere by jet-propelled aircraft.
Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
Suggest why the loss of ozone is an international environmental concern.
Nitric acid is usually produced by the oxidation of ammonia.
A mixture of nitric acid and sulfuric acid can be used to convert benzene to nitrobenzene, C6H5NO2.
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
Explain the relative lengths of the three bonds between N and O in nitric acid.
State a technique used to determine the length of the bonds between N and O in solid HNO3.
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
Draw the structural formula of the carbocation intermediate produced when this electrophile attacks benzene.
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
Benzoic acid, C6H5COOH, is another derivative of benzene.
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
Outline one piece of physical evidence for the structure of the benzene ring.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
The combustion reaction in (f)(ii) can also be classed as redox. Identify the atom that is oxidized and the atom that is reduced.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw two Lewis (electron dot) structures for BrO3−.
Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving your reasons.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
State and explain the magnetic property of iron(II) and iron(III) ions.
Hybridization of hydrocarbons affects their reactivity.
Experiments were carried out to investigate the mechanism of reaction between 2-chloropentane and aqueous sodium hydroxide.
Distinguish between a sigma and pi bond.
Identify the hybridization of carbon in ethane, ethene and ethyne.
State, giving a reason, if but-1-ene exhibits cis-trans isomerism.
State the type of reaction which occurs between but-1-ene and hydrogen iodide at room temperature.
Explain the mechanism of the reaction between but-1-ene with hydrogen iodide, using curly arrows to represent the movement of electron pairs.
State, giving a reason, if the product of this reaction exhibits stereoisomerism.
Deduce the rate expression for this reaction.
Deduce the units of the rate constant.
Determine the initial rate of reaction in experiment 4.
Deduce, with a reason, the mechanism of the reaction between 2-chloropentane and sodium hydroxide.
Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
Propane and propene are members of different homologous series.
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi () bonds are formed.
Deduce the number of σ and bonds in a molecule of ethyne.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
State the name of Compound B, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Compound A and Compound B are both liquids at room temperature and pressure. Identify the strongest intermolecular force between molecules of Compound A.
State the number of (sigma) and (pi) bonds in Compound A.
Deduce the hybridization of the central carbon atom in Compound A.
Identify the isomer of Compound B that exists as optical isomers (enantiomers).
Draw the structural formula of the alkene required.
Explain why the reaction produces more (CH3)3COH than (CH3)2CHCH2OH.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
A compound with a molecular formula C7H14O produced the following high resolution 1H NMR spectrum.
Deduce what information can be obtained from the 1H NMR spectrum.
Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of this compound using section 26 of the data booklet and the 1H NMR.
Suggest the structural formula of this compound.
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
State the reagents and the name of the mechanism for the nitration of benzene.
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Predict the bond angle in the ozone molecule.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Identify the steps which absorb ultraviolet light.
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor vehicle engines, and has the following Lewis structure.
Show how nitrogen monoxide catalyses the decomposition of ozone, including equations in your answer.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.

Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.

Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
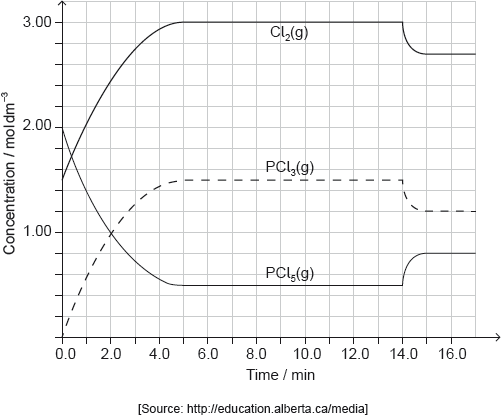
Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion.
Draw the structural formula of ethoxyethane
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) ⇌ NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
Draw a Lewis (electron dot) structure of chloramine.
State the hybridization of the nitrogen atom in chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
State the type of bond formed when chloramine is protonated.
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.